Abstract
Introduction:
Obesity is a known risk factor for development and worsening of venous thromboembolic episodes (VTEs). The 2016 International Society of Thrombosis and Hemostasis (ISTH) guidelines recommend caution using direct-acting oral anticoagulants (DOACs) in patients with morbid obesity (BMI >40 kg/m2 or weight > 120 kg) due to limited clinical efficacy and safety data supporting their use in this population. The ISTH recommends laboratory monitoring and dose adjustment as needed, if DOACs are being used in these patients. Unfortunately, most health systems do not have timely access to these tests, and the laboratory monitoring of DOACs remains a non-validated concept. Although the review of the currently available data does not reveal a significantly higher risk of recurrent VTE when using DOACs in these patients, the data is limited due to their retrospective and non-randomized nature and are thus hypothesis generating. We hypothesize that recurrent VTEs are higher in patients with morbid obesity and sought to investigate the differences in VTE recurrences in these patients when compared to the other patients.
Methods:
After institutional review board approval, we conducted a retrospective chart review for 1 year period (Jan 1, 2020 - Dec 31, 2020) on all patients >/= 18 years of age who were seen at our institution, who received a DOAC (Apixaban, Rivaroxaban, Edoxaban or Dabigatran). Patients who did not have any documentation of the outcome of interest, and those who were non-compliant with the DOACs were excluded. In the remainder of the cohort, data on BMI and recurrence of VTE after starting DOACs was collected and analyzed. Fisher's exact test was used to compare VTE recurrence in the different BMI groups. VTE recurrence in BMI groups taking the same DOAC was also compared individually. SPSS version 28 was used for statistical analysis.
Results:
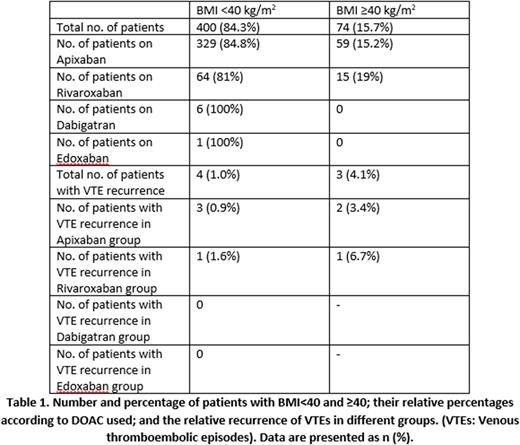
A total of 549 patients were initially collected and reviewed. 15 patients who did not have any documentation regarding VTE recurrence were excluded. 60 patients who were non-compliant with DOACs were excluded as well. The remaining 474 were including in the final study, out of which 288 (60.7%) were female, and 186 (39.3%) were male. Median age was 60, ±24. 400 (84.3%) had BMI <40 kg/m2, and 74 (15.7%) had BMI ≥40 kg/m2. 388 (81.9%) patients were on Apixaban, 6 (1.2%) on Dabigatran, 79 (16.7%) on Rivaroxaban, and 1 (0.2%) on Edoxaban. Recurrence of VTE while on the DOAC was observed in 4 (1%) patients with BMI <40 kg/m2, compared to 3 (4.1%) patients with ≥40 kg/m2 (p=0.080). Among those taking Apixaban, 3 (0.9%) patients with BMI <40 kg/m2 had recurrence, compared to 2 (3.4%) with ≥40 kg/m2 (p=0.16). For those on Rivaroxaban, 1 (1.6%) patient with BMI <40 kg/m2 had recurrence, compared to 1 (6.7%) patient with ≥40 kg/m2 (p=0.346). No recurrences were observed in patients on Dabigatran and Edoxaban. [Table 1]
Conclusions:
There was a numerically higher but statistically insignificant increased risk of recurrent VTE while on a DOAC in patients with BMI ≥40 kg/m2 compared to those with BMI <40 kg/m2 (4.1% vs 1%). This increased risk was similar with comparison of individual DOACs as well (3.4% vs 0.9% in the Apixaban group; 6.7% vs 1.6% in the Rivaroxaban group). Our study did not achieve statistical significance to prove our hypothesis, possibly because the analysis was underpowered. Further prospective studies with a larger sample size are needed to establish the safety of DOACs in morbidly obese patients.
Disclosures
Master:Cardinal Health: Membership on an entity's Board of Directors or advisory committees; Aptitude health: Membership on an entity's Board of Directors or advisory committees; curio sciences: Membership on an entity's Board of Directors or advisory committees; Blue Bird Bio: Current holder of stock options in a privately-held company; Toxicity: Current holder of stock options in a privately-held company; Gilead: Current holder of stock options in a privately-held company; Jasper: Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.